ARGENE® Transplant Range
For Viral Infection Management in Transplant Patients
Our real-time PCR assays permit rapid and specific detection of various viral infections prior to viral diseases. This is of vital importance in the management of the transplant patients, to prevent rejection and to allow patient survival.
Disclaimer: Product availability varies by country. Please consult your local bioMérieux representative for product availability in your country.
- ARGENE Transplant Range
- Overview
- Assays
- Resources
Overview
Detecting Active Viral Infections
By using our quantitative real-time PCR assays to monitor patients, at predefined intervals after transplantation, clinicians can detect active infections before symptoms arise.
Optimized detection and monitoring of infection in immunocompromised patients is critical to ensure the best patient management. Our ARGENE® Transplant range offers rapid and specific detection even prior to clinical symptoms, making it an ideal solution. This helps improve options for management, measure the effectiveness of treatment, and monitor for relapse. For even more comprehensive testing, you can use the broad ARGENE® range to quantify different viruses in one sample or analyze various samples for one virus at the same time.
- Sensitive and reproducible
- Reliable measurement of viral infection
- Wide linear range
- Standardized
- Uniform processing with ARGENE® range of products & harmonized test profiles for multiple assays in one run
- Protocol to convert quantification results into IU/mL with the WHO 1st International Standard (only valid for some of the viruses detected by the transplant range)
- Flexible
- Validated for use with various sample types
- Use manual or automated sample preparation such as nucliSENS® easyMAG® and EMAG® and assay setup system such as ESTREAM® liquid handling platform
- Qualified with the major real-time PCR platforms
Everything You Need in 1 Kit
The ready-to-use R-GENE® molecular detection kit measures viral load in DNA extracts from different clinical samples. This 5’ nuclease-based real-time PCR assay amplifies a specific region of the viral genome for detection and quantification.
- Four Quantification Standards ensure accurate viral load measurement
- Sensitivity Control validates the performance of the assay
- An Internal Control (IC2) checks the extraction process, including lysis, and the presence of amplification inhibitors in the sample
- All necessary reagents optimized to detect and quantify viral infection for in vitro diagnostic use are ready to use:
- Less technician time
- Less risk of manipulation or dilution error
- Less risk of contamination
Easy Procedure
Using R-GENE® assays is so simple, all you need to do is add the sample extracted DNA to the ready-to-use PCR master mix and start the reaction on the appropriate Real-Time PCR thermocycler, following the optimized cycling program described in the “Instructions For Use” document.
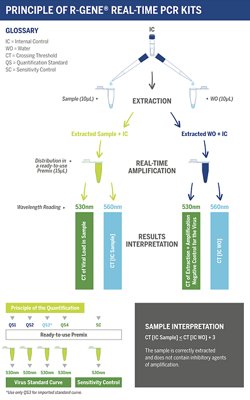
ARGENE® Expertise
- Simplicity: complete kits, ready-to-use reagents, same pipetting procedure
- Seamless Integration: validated for use on multi-specimens, multi-extraction and multi-amplification platforms
- Lab Efficiency: common internal control, harmonized extraction and amplification protocols, multiple targeted detection from one extracted sample
Assays
| KIT DESIGNATION | REFERENCES | TYPE OF KIT | NUMBER OF TESTS | REGULATORY STATUS* |
|---|---|---|---|---|
| EBV R-GENE® | 69-002B | Real-time detection and quantification kit | 90 | For In vitro diagnostic use (IVDR) |
| CMV R-GENE® | 69-003B | Real-time detection and quantification kit | 90 | For In vitro diagnostic use (IVDR) |
| HSV1&2 VZV R-GENE® | 69-014B | Real-time detection and quantification kit | 120 | For In vitro diagnostic use (IVDR) |
| ADENOVIRUS R-GENE® | 69-010B | Real-time detection and quantification kit | 90 | For In vitro diagnostic use (IVDR) |
| BK Virus R-GENE® | 69-013B | Real-time detection and quantification kit | 90 | For In vitro diagnostic use (IVDR) |
| Parvovirus B19 R-GENE® | 69-019B | Real-time detection and quantification kit | 90 | For In vitro diagnostic use (IVDR) |
| HHV6 R-GENE® | 69-006B | Real-time detection and quantification kit | 90 | For In vitro diagnostic use (IVDR) |
*IVDR: CE marked under EU regulation 2017/746
| EBV R-GENE® (69-002B) | |
|---|---|
| Principle of the test | Genomic detection and quantification of EBV |
| Ordering information | Reference 69-002B Designation: EBV R-GENE® Real-Time Detection and Quantification kit |
| Technology | Real-Time PCR / 5‘ nuclease Taqman technology |
| PCR design & Gene target | BXLF1 gene coding for thymidine kinase |
| Kit content | All included (Amplification premix, Internal control, Negative control, Quantification standards) |
Controls included | Extraction + Inhibition control, Sensitivity control, Negative control |
| Specimens |
|
| Dynamic Range of Quantification | From 500 copies/mL and 1.00E+07 copies/mL |
| Results within | 75 minutes (extraction step not included) |
| Reporting unit | Copies/mL or convert to IU/mL with WHO 1st International Standard |
| Validated Extraction platforms |
|
| Validated Amplification platforms |
|
Number of tests | 90 tests |
| Storage conditions | -15°C/-31°C |
| Status | For in vitro diagnostic use (CE marked under EU IVD regulation 2017/746) |
| CMV R-GENE® (69-003B) | |
|---|---|
| Principle of the test | Genomic detection and quantification of CMV |
| Ordering information | Reference 69-003B Designation: CMV R-GENE® Real-Time Detection and Quantification kit |
| Technology | Real-Time PCR / 5‘ nuclease Taqman technology |
| PCR design & Gene target | ppUL83 protein |
| Kit content | All included (Amplification premix, Internal control, Negative control, Quantification standards) |
Controls included | Extraction + Inhibition control, Sensitivity control, Negative control |
| Specimens |
|
| Dynamic Range of Quantification | 500 copies/mL and 7.2E+07 copies/mL |
| Results within | 75 minutes (extraction step not included) |
| Reporting unit | Copies/mL or convert to IU/mL with WHO 1st International Standard |
| Validated Extraction platforms |
|
| Validated Amplification platforms |
|
Number of tests | 90 tests |
| Storage conditions | -15°C/-31°C |
| Status | For in vitro diagnostic use (CE marked under EU IVD regulation 2017/746) |
| HSV1&2 VZV R-GENE® (69-014B) | |
|---|---|
| Principle of the test | Genomic detection and quantification of HSV-1, HSV-2 and VZV |
| Ordering information | Reference 69-014B Designation: HSV1&2 VZV R-GENE® Detection and Quantification kit |
| Technology | Real-Time PCR / 5‘ nuclease Taqman technology |
| PCR design & Gene target |
|
| Kit content | All included (Amplification premix, Internal control, Negative control, Quantification standards) |
Controls included | Extraction + Inhibition control, Sensitivity control, Negative control |
| Specimens | For qualitative and quantitative detection:
For qualitative detection:
|
| Dynamic Range of Quantification |
|
| Results within | 75 minutes (extraction step not included) |
| Reporting unit | Copies/mL or convert to IU/mL with WHO 1st International Standard |
| Validated Extraction platforms |
|
| Validated Amplification platforms |
|
Number of tests | 60 tests for HSV1 and HSV2, 60 tests for VZV |
| Storage conditions | -15°C/-31°C |
| Status | For in vitro diagnostic use (CE marked under EU IVD regulation 2017/746) |
| ADENOVIRUS R-GENE® (69-010B) | |
|---|---|
| Principle of the test | Genomic detection and quantification of ADENOVIRUS |
| Ordering information | Reference 69-010B Designation: ADENOVIRUS R-GENE® - Real-Time Detection and Quantification kit |
| Technology | Real-Time PCR / 5‘ nuclease Taqman technology |
| PCR design & Gene target | Hexon gene |
| Kit content | All included (Amplification premix, Internal control, Negative control, Quantification standards) |
Controls included | Extraction + Inhibition control, Sensitivity control, Negative control |
| Specimens |
|
| Dynamic Range of Quantification |
|
| Results within | 75 minutes (extraction step not included) |
| Reporting unit | Copies/mL or convert to IU/mL with WHO 1st International Standard |
| Validated Extraction platforms |
|
| Validated Amplification platforms |
|
Number of tests | 90 tests |
| Storage conditions | -15°C/-31°C |
| Status | For in vitro diagnostic use (CE marked under EU IVD regulation 2017/746) |
| BK Virus R-GENE® (69-013B) | |
|---|---|
| Principle of the test | Genomic detection and quantification of BK Virus |
| Ordering information | Reference 69-013B Designation: BK Virus R-GENE® - Real-Time Detection and Quantification kit |
| Technology | Real-Time PCR / 5‘ nuclease Taqman technology |
| PCR design & Gene target | Small T Antigen (StAg) |
| Kit content | All included (Amplification premix, Internal control, Negative control, Quantification standards) |
Controls included | Extraction + Inhibition control, Sensitivity control, Negative control |
| Specimens |
|
| Dynamic Range of Quantification |
|
| Results within | 75 minutes (extraction step not included) |
| Reporting unit | Copies/mL or convert to IU/mL with WHO 1st International Standard |
| Validated Extraction platforms |
|
| Validated Amplification platforms |
|
Number of tests | 90 tests |
| Storage conditions | -15°C/-31°C |
| Status | For in vitro diagnostic use (CE marked under EU IVD regulation 2017/746) |
| Parvovirus B19 R-GENE® (69-019B) | |
|---|---|
| Principle of the test | Genomic detection and quantification of Parvovirus B19 |
| Ordering information | Reference 69-019B Designation: Parvovirus B19 R-GENE® - Real-Time Detection and Quantification kit |
| Technology | Real-Time PCR / 5‘ nuclease Taqman technology |
| PCR design & Gene target | NS1 gene
|
| Kit content | All included (Amplification premix, Internal control, Negative control, Quantification standards) |
Controls included | Extraction + Inhibition control, Sensitivity control, Negative control |
| Specimens |
|
| Dynamic Range of Quantification | 500 copies/mL and 1.00E+10 copies/mL |
| Results within | 75 minutes (extraction step not included) |
| Reporting unit | Copies/mL or convert to IU/mL with WHO 1st International Standard |
| Validated Extraction platforms |
|
| Validated Amplification platforms |
|
Number of tests | 90 tests |
| Storage conditions | -15°C/-31°C |
| Status | For in vitro diagnostic use (CE marked under EU IVD regulation 2017/746) |
| HHV6 R-GENE® (69-006B) | |
|---|---|
| Principle of the test | Genomic detection and quantification of HHV6 |
| Ordering information | Reference 69-006B Designation: HHV6 R-GENE® - Real Time Detection and Quantification kit |
| Technology | Real-Time PCR / 5‘ nuclease Taqman technology |
| PCR design & Gene target | U57 gene |
| Kit content | All included (Amplification premix, Internal control, Positive & Negative control, quantification standards) |
Controls included | Extraction + Inhibition control, Sensitivity control, Negative control |
| Specimens |
|
| Dynamic Range of Quantification |
|
| Results within | 75 minutes (extraction step not included) |
| Reporting unit | Copies/mL or convert to IU/mL with WHO 1st International Standard |
| Validated Extraction platforms |
|
Validated Amplification platforms
|
|
Number of tests | 90 tests |
| Storage conditions | -15°C/-31°C |
| Status | For in vitro diagnostic use (CE marked under EU IVD regulation 2017/746) |