ETEST®
Antibiotic Susceptibility Testing Reagent Strips to Determine On-Scale MICs
Clinicians often need more information than what primary AST can provide. Recognized around the world for their proven performance, ETEST® ready to use reagent strips determine on-scale MICs.
Disclaimer: Product availability varies by country. Please consult your local bioMérieux representative for product availability in your country.
- Overview
- Equipments
- Specs & Resources
Overview
Proven Medical Value in the Face of Growing Resistance
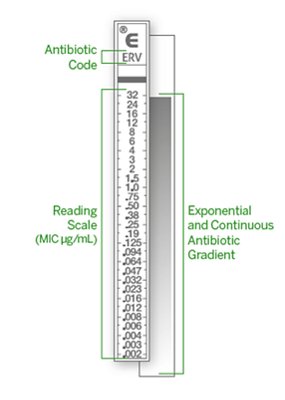
ETEST® strips – the original gradient MIC strips – are considered a gold standard by many. The strips certainly uphold their dependable reputation when it comes to establishing the on-scale Minimum Inhibitory Concentration (MIC) of antibiotics and antifungal agents. ETEST® is a predefined, stable gradient of 15 antimicrobial concentrations on a plastic strip. ETEST® has an extensive range of over 90 antimicrobial references available in the following categories: antibiotics, antifungals, and antimicrobial resistance detection (ARD).
ETEST® is a simple, cost-effective tool that offers MIC results to complement your routine antimicrobial susceptibility tests (AST) performed on VITEK® 2 systems, or other automated platforms or manual testing (Kirby-Bauer method) allowing you to:
- Test new/non-routine antimicrobials
- Determine the MIC of fastidious, or slow-growing micro-organisms
- Confirm or detect low-level or new resistance mechanisms
- Fine-tune antimicrobial treatment
The Trusted Original Gradient MIC Strips
Did you know?
Versatile & Flexible: The Method of Choice to Supplement Your Main Primary Routine AST System
ETEST® is the most accurate solution for completing the AST profile:
- For all new antimicrobials that are not accessible in a routine mode
- For difficult organisms that can’t be tested by primary AST methods (e.g., fastidious, anaerobic, primary AST limitations, etc.)
- For drug/bug combinations that are not available
ETEST® fills gaps that primary testing cannot cover:
- Detecting low levels of resistance or new resistance mechanisms
- Fine-tuning antimicrobials treatment (MDROs, drug with narrow therapeutic index, critically ill patients, breakpoints zone, or ATU)
- Detecting or confirming antimicrobial resistant phenotypes such as ESBL, MBL, AmpC*, or GISA/hGISA*
*For Research Use Only
Extended-Range MICs Spanning 15 Two-Fold Dilutions
ETEST® provides full-range MICs spanning 15 two-fold dilutions for concentrations that are ideal for refining patient treatment in instances where routine AST methods are limited in one of the following ways:
- Provides S, I, or R category results only
- MIC results are off-scale (i.e., “<” or “>” results) and do not provide sufficient data for pharmacokinetic/pharmacodynamic (PK-PD) targeted dosing considerations
Easy-to-Use MIC Technique
ETEST® is easy to implement and use:
- No software or specific skills required
- Optional equipment available to standardize plate preparation and strip application
- Individual testing – perfectly adapted for MIC testing for newly launched antimicrobials
Equipments
ETEST® Equipments Designed to Standardize Your Setup and Save Time
Save time and increase reproducibility with ETEST® equipment and accessories designed to simplify the daily use of ETEST®.

| ETEST® Equipment | Reference | Description |
|---|---|---|
| Simplex C76™ | Ref. 559802 | Automated applicator to rapidly apply ETEST® strips to agar plates in optimal, predetermined patterns.
|

| ETEST® Equipment | Reference | Description |
|---|---|---|
| Retro C80™ | Ref. 559803 | Rota-plater to optimize plate inoculation.
|

| ETEST® Equipment | Reference | Description |
|---|---|---|
| Nema C88™ | Ref. 559804 | Vacuum pen to aspirate ETEST® strip and position it on agar – simplifies the application of ETEST® strips on plate. |

| ETEST® Equipment | Reference | Description |
|---|---|---|
| Mini Grip-It | Ref. 411200 | Manual applicator to pick up ETEST® strips. |
Specs & Resources
Specifications
ETEST® Strips:
- Over 90 antimicrobials in the following categories: Antibiotics, Antifungals, and Antimicrobial Resistance Detection (ARD)
- Easy to implement in most clinical laboratories. Ready-to-use AST
- Predefined, and stable antimicrobial gradient (dry chemistry)
- Inert, non-porous plastic strip
- Agar-based method with its advantages (e.g.: identify contamination...)
- Large scale (15 antimicrobial concentrations)
- Half dilution, accurate on-scale MICs value
- Performances validated against a reference method
ETEST® Packaging Designed by bioMérieux to Suit Your Needs
SINGLE PACK: 30 Strips
- Individual strips packaging, easy opening, adapted to the low testing volume
MULTIPACK: 100 Strips
- 10 cartridges of 10 strips, perfectly adapted to high-volume testing
- Cartridge compatible with the APPLICATOR SIMPLEX C76™

Resources
Testimonials
“ETEST® is well established in our department. It has become a rapid and accurate tool for the determination of MICs in clinically relevant and urgent situations, e.g., for pathogens from blood cultures or CSF. It is also great to have in other clinical situations where it is important to know "how susceptible or resistant" the pathogen is. Also, for fungi, ETEST® is just fabulous. What a pain when MICs had to be determined by dilution methods!”
“On behalf of all my patients, who have benefitted from ETEST® MIC testing, thank you! You've helped save the lives of patients with serious bacterial infections all over the world.”
“ETEST® makes possible a standard method for testing a myriad of different types of organisms, almost "one-stop shopping". This has allowed laboratories with varying levels of expertise to perform antimicrobial susceptibility testing with an enormous positive impact on patient care. Having this technology available even in resource-poor areas of the world, has contributed to the detection of resistances where such knowledge was previously unattainable.”