VIDAS® NEPHROCHECK®
Reveal Kidney Stress Early. Drive Better Outcomes.
VIDAS® NEPHROCHECK® is an automated test for use on the VIDAS® 3 instrument for the immunoenzymatic quantitative determination of TIMP-2 (Tissue Inhibitor of Metalloproteinase-2) and IGFBP-7 (Insulin-like Growth Factor-Binding Protein 7) proteins in human urine using the ELFA technique (Enzyme Linked Fluorescent Assay).
The VIDAS® NEPHROCHECK® assay is intended to be used in conjunction with clinical evaluation as an aid in the risk assessment for moderate or severe acute kidney injury (AKI) in acutely ill patients.
Disclaimer: Product availability varies by country. Please consult your local bioMérieux representative for product availability in your country

- VIDAS NEPHROCHECK
- Overview
- Assay
- Resources
Overview
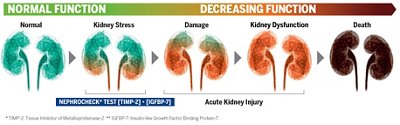
AKI is one of the most common syndromes in ICU patients and there is no direct treatment.
Every day, clinicians make important decisions to save patients’ lives. Aggressive treatments may be needed, which sometimes include nephrotoxic agents. When limited information is available to monitor the kidney status, they may lead to a rapid loss of kidney function (typically within 48 hours).
Commonly used indicators, e.g. serum creatinine and urine output, are known to be lagging:
- They may be normal when kidney damage has already occurred.
- They can be complex to measure and interpret.
Today, a test detects kidney stress before the damage occurs, when intervention can still make a difference.
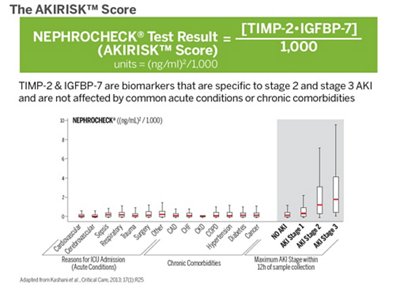
Clinical utility of VIDAS® NEPHROCHECK®
Reveal Kidney Stress Earlier
VIDAS® NEPHROCHECK® aids to:
- Identify patients at risk of moderate or severe AKI within 12 hours of assessment,
- De-escalate monitoring with confidence and adapt treatment using goal-directed protocols
Drive Better Outcomes
With the results given by the VIDAS® NEPHROCHECK® test, clinicians may:
- Want to implement early renal-protective actions and reduce the incidence of moderate and severe AKI,
- Optimize time and resources potentially leading to reduced ICU length of stay and extra costs.
Thanks to the high sensitivity of VIDAS® NEPHROCHECK®, important decisions for acutely ill patients can be done with a higher degree of confidence:
VIDAS® Solutions
- Reliable and easy-to-use instruments with random access and small foot-print.
- Well adapted to rapid response laboratories.
- Factory-calibrated, single-dose tests which reduce the need for additional controls.
- Short time to result.
- Reagents can be used immediately after removal from the refrigerator.
Assay
Technical Specifications
| Assay | Reference | Tests per Kit | Code | Time to Result | Measuring Range |
|---|---|---|---|---|---|
| VIDAS® NEPHROCHECK® | 421172-03 | 30 tests | NEPH | 46 minutes | 0.04 - 10.00 AKIRISK™ Score |
BECAUSE IT MAKES SENSE ON VIDAS®