VIDAS® C. difficile Panel
Rapid Identification of C. difficile Infections
The VIDAS® C. difficile Panel includes two complementary tests that aid in the diagnosis of C. difficile infection.
Disclaimer: Product availability varies by country. Please consult your local bioMérieux representative for product availability in your country.
- Overview
- Assays
- Resources
Overview
VIDAS® C. difficile GDH is a qualitative test that detects the C. difficile antigen, glutamate dehydrogenase (GDH), in stool specimens to screen patients suspected of having C. difficile infection. It is used in conjunction with VIDAS® C. difficile Toxin A&B as part of a two-step algorithm.
VIDAS® C. difficile immunoassays are adapted to current diagnosis guidelines with:
- VIDAS® GDH highly sensitive test for the screening
- VIDAS® CDAB highly specific test for the toxin detection
C. difficile Infection Management
Clostridioides difficile (C. difficile) is one of the most challenging Healthcare Associated Infections (HAI)1. It is usually contracted in the healthcare setting and is connected to antibiotic treatment. C. difficile infection (CDI) generates costly treatment, patient isolation, and longer hospital stays1.
The rise of Community Acquired Infections is of concern and now accounts for 35-48% of CDI diagnosis in the United States2. During a point-prevalence study in Europe, the CDI positivity was found to be at 1.3% in community samples, and half of community CDI cases were undetected because of the absence of clinical suspicion, representing 3 times more undiagnosed adults in the community compared with hospitals2.
Continued appropriate infection control, antibiotic use, and diagnostic testing are important in decreasing C. difficile cases3.
Greater Clinical Value for Clinicians
The VIDAS® C. difficile panel helps improve patient management with actionable results based on excellent clinical performance:
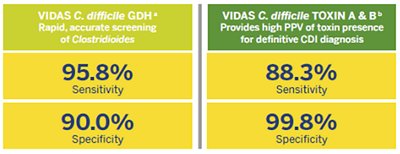
aCompared to Bacterial Culture CCFA. bCompared to Cellular Cytotoxicity Assay.
Source: VIDAS® C. difficile GDH and VIDAS® C. difficile TOXIN A & B package inserts.
VIDAS® Solutions
- Reliable and easy-to-use instruments with random access and small footprint.
- Well adapted to rapid response laboratories.
- Factory-calibrated, single-dose tests which reduce the need for additional controls.
- Short time to result.
References
1. CDC’s 2019 Antibiotic Resistance Threats Report.
2. kelly, C.R., et al. (2021). “ACG Clinical Guidelines: Prevention, Diagnosis, and Treatment of Clostridioides difficileInfections.” Am J Gastroenterol 116(6):1124-1147.
3. Viprey, V.F., et al. (2022). “A point-prevalence study on community and inpatient Clostridioides difficile infections (CDI): results from Combatting Bacterial Resistance in Europe CDI (COMBACTE-CDI), July to November 2018.” Euro Surveill 27(26).
Assays
Technical Specifications
| Assay | Reference | Code | Tests per Kit | Time to Result | Measurement Range or Interpretation |
|---|---|---|---|---|---|
| VIDAS® C. difficile TOXIN A & B | 30118 | CDAB | 60 | 75 min | Qualitative test : Negative or positive |
| VIDAS® C. difficile GDH | 30125 | GDH | 60 | 50 min | Qualitative test : Negative or positive |
BECAUSE IT MAKES SENSE ON VIDAS®