VIDAS® High Sensitive Troponin I
Optimize the Management of Acute Coronary Syndrome (ACS)
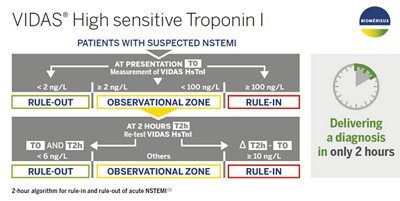
The VIDAS® High sensitive Troponin I assay is intended to be used as an aid in the diagnosis of myocardial infarction delivering actionable results in only 2 hours and for the risk stratification of adult patients with acute coronary syndrome.
Disclaimer: Product availability varies by country. Please consult your local bioMérieux representative for product availability in your country.
- Overview
- Assay
- Resources
Overview
VIDAS® High sensitive Troponin I is an automated quantitative test for use on the instruments of the VIDAS® family for the determination of human cardiac troponin I in human serum or plasma, using the ELFA technique (Enzyme Linked Fluorescent Assay). The VIDAS® High sensitive Troponin I assay is intended to be used as an aid in the diagnosis of myocardial infarction (MI) and for the risk stratification of patients with symptoms suggestive of acute coronary syndrome (ACS) with respect to relative risk of all‑cause mortality and major adverse cardiac events (MACE) consisting of myocardial infarction and revascularization, at 30 days.
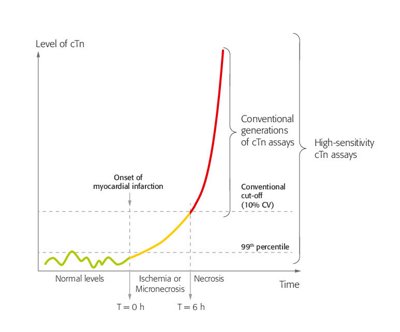
The troponin complex consists of 3 subunits (I, T and C) and is essential for the regulation of skeletal and cardiac muscle contraction.1 In contrast to troponin C, cardiac-specific isoforms of troponin T and I exist. The release of cTnI and cTnT from cardiomyocytes is highly specific for myocardial injury. However, any type of injury, not just ischemic injury, can result in the release of cTn into the blood circulation.2 Consequently, cTn can also be elevated in other conditions than MI.
Specific antibodies have been raised against the cardiac-specific cTn isoforms, the basis of widely available quantitative cTnI and cTnT assays.3 Advances in technology have led to an improvement of the ability of cTn assays to detect and quantify cardiomyocyte injury. Because of their increased analytical power, allowing accurate measurement below the 99th percentile URL as well as accurate measurement of small absolute changes, high-sensitivity cTn (hs-cTn) assays enable earlier detection of AMI with shortening of the time window for serial testing.4-6
VIDAS® Solutions
- Reliable and easy-to-use instruments with random access and small footprint.
- Well adapted to rapid response laboratories.
- Factory-calibrated, single-dose tests which reduce the need for additional controls.
- Short time to result.
- Reagents can be used immediately after removal from the refrigerator.
References
1. Parmacek MS, Solaro RJ. Biology of the troponin complex in cardiac myocytes. Prog Cardiovasc Dis. 2004; 47:159-76.
2. Thygesen K, Alpert JS, Jaffe AS, et al.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018;138:e618-e651.
3. Apple FS, Sandoval Y, Jaffe AS, Ordonez-Llanos J; IFCC Task Force on Clinical Applications of Cardiac Bio-Markers. Cardiac troponin assays: guide to understanding analytical characteristics and their impact on clinical care. Clin Chem. 2017;63:73-81.
4. Apple FS, Collinson PO; IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem. 2012;58:54-61.
5. Thygesen K, Mair J, Giannitsis E, et al.; Study Group on Biomarkers in Cardiology of ESC Working Group on Acute Cardiac Care. How to use high-sensitivity cardiac troponins in acute cardiac care.Eur Heart J. 2012;33:2252-2257.
6. Al-Saleh A, Alazzoni A, Al Shalash S, et al. Performance of the high-sensitivity troponin assay in diagnosing acute myocardial infarction: systematic review and meta-analysis. CMAJ Open. 2014;2:E199-207
Assay
Technical Specifications
| Assay | Reference | Tests per Kit | Code | Test Time (Minutes) | Measuring Range |
|---|---|---|---|---|---|
| VIDAS® High Sensitive Troponin I | 415386 415386-30 | 60 tests 30 tests | TNHS | 20 | 1.5-40,000 pg/mL |
BECAUSE IT MAKES SENSE ON VIDAS®

Resources