VIDAS® Arboviruses Panel
Suspecting an Arbovirus? Think Dengue and Chikungunya.
Our VIDAS® offer of immunoassays directed against arboviruses diseases improve access to medically important automated diagnostic tests.
Disclaimer: Product availability varies by country. Please consult your local bioMérieux representative for product availability in your country.
- Overview
- Assays
- Resources
Overview
Dengue & Chikungunya are among the most important diseases caused by arboviruses worldwide, especially in tropical and subtropical regions. These are transmitted to humans by day-biting Aedes aegypti and Aedes albopictus mosquitoes.
VIDAS® range offers 2 test panels: VIDAS® DENGUE panel (NS1 Ag, Anti-Dengue IgM, Anti-Dengue IgG) and VIDAS® CHIKUNGUNYA panel (Anti-CHIKUNGUNYA IgM, Anti-CHIKUNGUNYA IgG)
VIDAS® DENGUE PANEL
According to WHO estimates, the dengue virus infects 100 to 400 million people worldwide every year, and it is rapidly spreading. A leading cause of serious illness and death in Asia and Latin America, dengue now affects new areas, including Europe. Early detection of disease progression associated with severe dengue is essential to reduce fatality rates. However, symptoms are not specific, and can be easily confused with other infection diseases such as malaria, chikungunya, Zika, or yellow fever.
The VIDAS® DENGUE panel includes 3 serological tests recommended by international recommendations for precise and early diagnosis. Accessible to all laboratories, they allow rapid and reliable confirmation (or exclusion) of dengue infection for optimized patient care.
A complete solution for dengue diagnosis with 3 independent & complementary tests:
NS1: The NS1 antigen is an early and sensitive viral marker of acute infection. It is the ideal solution for laboratories without access to RT-PCR, to detect the virus.
IgM: If IgM appears earlier than IgG, it also disappears earlier. This makes IgM an early marker of acute primary infection.
IgG: IgG is used to determine the presence of specific immunity.
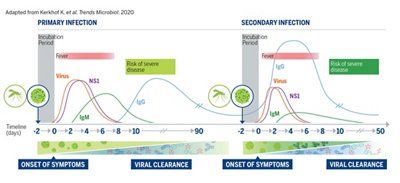
Kinetics of Dengue virus (genome/NS1 antigen) and antibodies (IgM & IgG)
During the early phase of the disease, direct diagnostic techniques such as NS1 are preferred. Moreover, combining NS1 detection with IgM serology in routine diagnosis of dengue virus infection can help improve accuracy of dengue diagnosis.
- Importance to combine NS1 and IgM and eventually IgG (for secondary infection)
In Post-acute phase, after 5-7 days post onset of symptoms, serological tests based on the patient’s immune response to infection (IgM and/or IgG) are commonly used4.
- Importance to combine IgM & IgG
With VIDAS®, choose your own combination of tests.
Proven High Performance
Based on our strong expertise in Infectious Diseases, the VIDAS® DENGUE panel offers high-quality reagents for reliable and clear-cut results:

VIDAS® NS1 Ag, VIDAS® Anti-DENGUE IgM, VIDAS® Anti-DENGUE IgG. Package inserts
Excellent performance on all dengue virus serotypes for confirmation or exclusion of dengue infection. No equivocal zone
The clinical study has been performed with samples from endemic populations below in Latin America, Africa, and Asia. In conclusion, the performance in the package inserts is representative of the most endemic areas.
VIDAS® CHIKUNGUNYA PANEL
Transmitted to humans through the bites of infected mosquitoes, chikungunya virus (CHIKV) was first identified in Tanzania in 1952. Over the last two decades it has spread across the world to more than 100 countries, causing regular outbreaks in Asia and Africa, massive outbreaks in the Americas, and more sporadic and clustered cases in Europe. Chronic chikungunya arthritis has emerged as an important viral infection causing debilitating chronic rheumatic disease throughout the world.
As symptoms of chikungunya (fever, headache, muscle pain, joint swelling, rash or joint pain) overlap with those of other mosquito-borne diseases, such as dengue and malaria, differential diagnosis in endemic areas is essential for optimized patient management. The VIDAS® CHIKUNGUNYA panel offers a complete solution for rapid and reliable confirmation of chikungunya infection.
Detection from Acute to Chronic Disease
Recommended Diagnostic Methods (1)
3 specific chikungunya biomarkers are expressed after infection:
- The presence of virus in the blood (viremia) can be detected early in the course of infection, and up to 7-10 days after the first symptoms.
- Anti-CHIKUNGUNYA IgM antibodies are generally detectable 5-7 days after the contamination. It is classically admitted that they persist for several weeks, up to 3 months. However, it has been demonstrated that they can be detected in the blood of infected people up to more than one year after the acute phase.
- Anti-CHIKUNGUNYA IgG antibodies are detectable a few days after IgM appearance, 7-10 days after the contamination, and can persist for years.
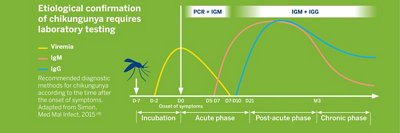
2 Complementary Tests
The VIDAS® CHIKUNGUNYA panel offers 2 complementary tests for accurate detection of the disease from the acute to the chronic phase:
- VIDAS® Anti-CHIKUNGUNYA IgM: Early serological response
- VIDAS® Anti-CHIKUNGUNYA IgG: Presence of specific immunity
Reliable and Clear-Cut Results
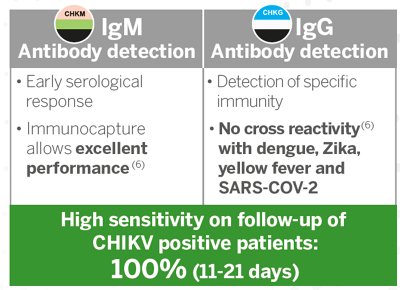
VIDAS® Anti-CHIKUNGUNYA IgM and IgG package inserts.
Developed with the contribution of the United States National Institute of Allergy and Infectious Diseases (NIAID), Vaccine Research Center (VRC)
VIDAS® Solutions
- Reliable and easy-to-use instruments with random access and small footprint.
- Well adapted to rapid response laboratories.
- Factory-calibrated, single-dose tests which reduce the need for additional controls.
- Short time to result.
References
INTERNATIONAL GUIDELINES
The Causes and Threats of Emerging and Re-emerging Arboviral Diseases https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10422058/
Pan American Health Organization (PAHO) guidelines for Dengue patient care in the Region of the Americas https://iris.paho.org/handle/10665.2/31207
WHO Dengue guidelines for diagnosis, treatment, prevention and control https://apps.who.int/iris/handle/10665/44188
CDC Testing guidance https://www.cdc.gov/dengue/healthcare-providers/testing/testing-guidance.html
Chronic chikungunya arthritis and rheumatoid arthritis: what they have in common - PMC https://www.nih.gov/
USEFUL LINKS
WHO | Dengue factsheet https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue
ECDC | Dengue factsheet https://www.ecdc.europa.eu/en/dengue-fever/facts/factsheet
Assays
Technical Specifications
| Assays | Reference | Code | Tests Per Kit | Time to Result | Decisional Cut-offs |
|---|---|---|---|---|---|
| VIDAS® DENGUE NS1 Ag | 423077 | DEAG | 60 tests | 60 minutes | Qualitative test : Negative or positive |
| VIDAS® Anti-DENGUE IgM | 423078 | DENM | 60 tests | 40 minutes | Qualitative test : Negative or positive |
| VIDAS® Anti-DENGUE IgG | 423079 | DENG | 60 tests | 40 minutes | Qualitative test : Negative or positive |
| VIDAS® Anti-CHIKUNGUNYA IgM | 423229-30 | CHKM | 30 tests | 40 minutes | Qualitative test : Negative or positive |
| VIDAS® Anti-CHIKUNGUNYA IgG | 423230-30 | CHKG | 30 tests | 40 minutes | Qualitative test : Negative or positive |
BECAUSE IT MAKES SENSE ON VIDAS®

Resources


