VIDAS® D-DIMER EXCLUSION™ II
Safe Exclusion of Venous Thromboembolism
VIDAS® D-DIMER EXCLUSION™ II is a highly-sensitive automated D-Dimer assay. Used in conjunction with assessment of clinical pretest probability, it allows safe exclusion of deep vein thrombosis (DVT) and pulmonary embolism (PE) in both low and intermediate suspected outpatient risk groups in just 20 minutes.
Disclaimer: Product availability varies by country. Please consult your local bioMérieux representative for product availability in your country.

- Overview
- Assay
- Resources
Overview
VIDAS® D-DIMER EXCLUSION™ II is an automated test for the immunoenzymatic determination of fibrin degradation products in human plasma. It is indicated for use in conjunction with a clinical pretest probability assessment model to exclude deep vein thrombosis (DVT) and pulmonary embolism (PE) disease in outpatients suspected of DVT or PE.
VIDAS® D-DIMER EXCLUSION™ II is the only test validated for use in the HERDOO2 clinical decision rule (CDR) to assess the risk of recurrence of venous thromboembolism (VTE) in women with a first unprovoked VTE. Risk stratification by this CDR is an aid to guide the duration of oral anticoagulant therapy.
D-dimer reflects the presence of stabilized fibrin and this has made this marker a useful tool in the diagnosis of venous thromboembolism (VTE).1 Quantitative D-dimer assays based on ELISA techniques have a high sensitivity for the presence of an occluding thrombus and, consequently, are particularly useful in excluding venous thromboembolism.2, 3 In conjunction with assessment of clinical pretest probability, it is possible to safely rule out the diagnosis of deep vein thrombosis (DVT) and/or pulmonary embolism (PE) in suspected outpatients when the concentration of D-dimer is below a predefined cutoff (determined by rigorous clinical studies).3, 4, 5
The clinical utility of D-dimer ELISA assays in the diagnostic work-up of suspected DVT or PE resides in the significant reduction in the number of imaging tests that are required, with a concomitant reduction in the total cost of diagnosis.6, 7 For patients with suspected pulmonary embolism, the use of an age-adjusted cutoff compared to a single clinical cutoff, enables to reduce the number of imaging tests required.8 D-dimer is not specific for DVT/PE and elevated levels are also observed in a variety of other conditions where activation of coagulation and fibrinolysis occurs (for example, surgery, trauma, infection, inflammation, pregnancy, cancer).1 This makes D-dimer less useful for exclusion of DVT or PE in hospitalized patients due to the high proportion of comorbid conditions associated with elevated D-dimer levels.3, 9 Under certain conditions, lower than expected D-dimer results may occur giving rise to false-negatives. Therefore, it is not safe to use D-dimer for exclusion of DVT/PE in patients with high pre-test probability, long duration of DVT/PE symptoms (more than one week), or already under anticoagulant treatment.9, 10
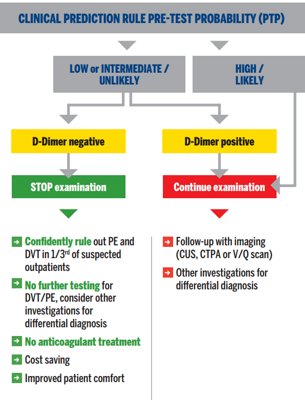
Diagnostic algorithm for suspected DVT or PE in outpatients3, 4, 15
- Hyperpigmentation, Edema, or Redness (HER) on examination of legs
- D-dimer ≥ 250 ng/mL
- Obesity (body mass index ≥ 30 kg/m2)
- Older age (≥ 65 years)
The International Society on Thrombosis and Haemostasis (ISTH) suggests that it is safe to discontinue anticoagulants if the risk of recurrent VTE is less than 5% one year after stopping anticoagulant therapy.12 The REVERSE II study confirmed that the one-year VTE recurrence rate was below the level recommended by the ISTH, since the recurrence rate in women with a first unprovoked VTE and 0 or 1 HERDOO2 criteria was 3.0% in this study.13
VIDAS® Solutions
- Reliable and easy-to-use instruments with random access and small footprint.
- Well adapted to rapid response laboratories.
- Factory-calibrated, single-dose tests which reduce the need for additional controls.
- Short time to result.
- Reagents can be used immediately after removal from the refrigerator.
References
1. BOCKENSTEDT P. - D-dimer in venous thromboembolism. - N Engl J Med.-2003;349:1203-4.
2. DI NISIO M, SQUIZZATO A, RUTJES AW, BÜLLER HR, ZWINDERMAN AH, BOSSUYT PM. - Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. - J Thromb Haemost. - 2007;5:296-304.
3. RIGHINI M, PERRIER A, DE MOERLOOSE P, BOUNAMEAUX H. - D-Dimer for venous thromboembolism diagnosis: 20 years later. - J Thromb Haemost. - 2008;6:1059-71.
4. TEN CATE-HOEK AJ, PRINS MH. - Management studies using a combination of D-dimer test result and clinical probability to rule out venous thromboembolism: a systematic review. - J Thromb Haemost. - 2005;3:2465-70.
5. CARRIER M, RIGHINI M, DJURABI RK, HUISMAN MV, PERRIER A, WELLS PS, RODGER M, WUILLEMIN WA, LE GAL G. - VIDAS D-dimer in combination with clinical pre-test probability to rule out pulmonary embolism. A systematic review of management outcome studies. - Thromb Haemost. - 2009;101:886-92
6. GOODACRE S, SAMPSON F, STEVENSON M, WAILOO A, SUTTON A, THOMAS S, LOCKER T, RYAN A. Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis. Health Technol Assess. 2006;10:1-168, iii-iv.
7. RIGHINI M, NENDAZ M, LE GAL G, BOUNAMEAUX H, PERRIER A. - Influence of age on the cost-effectiveness of diagnostic strategies for suspected pulmonary embolism. - J Thromb Haemost. - 2007;5:1869-77.
8. RIGHINI M, et al. - Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. - JAMA. - 2014;311:1117-24.
9. BRUINSTROOP E, VAN DE REE MA, HUISMAN MV. - The use of D-dimer in specific clinical conditions: a narrative review. - Eur J Intern Med. - 2009;20:441-6.
10. HOUDIJK W. - Proper observation of patient-related factors is an important determinant in the use of the D-dimer test for exclusion of venous thromboembolism in the ED. - Am J Emerg Med. - 2007;25:255-256.
11. RODGER MA, et al. - Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. - CMAJ. - 2008;179:417-26
12. KEARON C, IORIO A, PALARETI G, - Subcommittee on Control of Anticoagulation of the SSC of the ISTH. Risk of recurrent venous thromboembolism after stopping treatment in cohort studies: recommendation for acceptable rates and standardized reporting. - J Thromb Haemost. - 2010;8:2313-5.
13. RODGER et al. - Validating the HERDOO2 rule to guide treatment duration for women with unprovoked VTE
14. Huisman MV, Klok FA. Diagnostic management of acute deep vein thrombosis and pulmonary embolism. J Thromb Haemost 2013;11:412–22
15. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033-69, 3069a-3069k
Assay
Technical Specifications
| Assay | Reference | Tests per Kit | Code | Time to Result | Measuring Range |
|---|---|---|---|---|---|
| VIDAS® D-DIMER EXCLUSION™ II | 30455-02 30455-30 | 60 tests 30 tests | DEX2 | 20 minutes | 45 - 10,000 ng/mL (FEU) |
BECAUSE IT MAKES SENSE ON VIDAS®

Resources